SEARCH
Enter your search term below:
Close
Enter your search term below:

WORLD LEADING BUSINESS SUPPORT
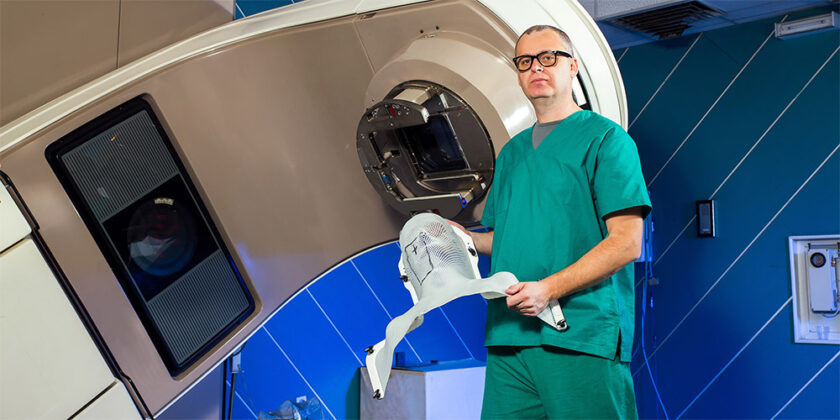
TRUEinvivo is revolutionising the success of radiotherapy treatments through its use of specially designed micro silica beads that can measure the exact dose of radiation a patient receives. The many advantages of this include better targeting of cancers, high confidence in dose accuracy, and a significant reduction in the damage of healthy tissue around the cancer. TRUEinvivo’s function does not just stop at medical applications however, the DOSEmapper™ technology has also been successfully used in nuclear areas, industrial applications, animal health, aerospace, blood banks and food irradiation.

Innovation to Commercialisation of University Research (ICURe)
Funding: Grants: £478k; Equity: £550k
2013
Took part in ICURe
2015
£5k Innovation voucher from InnovateUK recieved
2015
£28k SIRIUS award from UKTI
2015
£35k from ICURe programme
2016
£50k Women in Innovation grant from InnovateUK
2017
Science proven in 20 hospitals
March 2017
First patent published
2018
£180k Biomedical catalyst award recieved
August 2019
Second patent published
September 2019
Beads accepted CE class 1 medical device
October 2019
£430k seed funding round closed
November 2019
Pre-production reader
January 2020
DOSEmapper™ & Automated Reader Patent granted in USA
October 2020
£180k Innovate UK sustainability award received
January 2021
Patents granted in Europe
2021
£213k investment raised
2022
£170k investment raised
2023
£167k investment raised
May 2023
Looking to raise £1.2m-£2.5m in investment

“I applied for the ICURe programme because I had discovered that silicon beads could be excellent radiation detectors with high precision dosimetry for radiotherapy patients. ICURe provided the help I needed to understand how I should go about commercialising my research. ICURe really opened my eyes beyond academia.”
“With the funding from ICURe I was able to travel to all of the big conferences and radiotherapy centres around the world and talk to both customers and competitors. It gave me a deep understanding of the reality of the market far beyond everything I thought I knew from research papers.”
“Following ICURe I started doing more and more research trials in hospitals and clinical settings, publishing papers and presenting in conferences, because that’s the way a medical physicist accepts change. Currently in advanced countries, up to 30% of patients do not receive the right dose of radiotherapy within recommended accuracy of ±5%, which causes damaging side effects and failure of the treatment. Our technology has an accuracy of +/- 3%, which is game changing.”
“We grew the team to 7 people, bringing in these key hires to fill the commercial side of the business allowed us to secure the funding we needed. We have finished our prototypes and we need to get them to industrial quality standard and mass production. Then we can offer our measuring beads and the automated reader as a pair to hospitals, who can then start to use them autonomously.”
“We are now a team of 3.5 people as we look to secure investment (£1.2m – £2.5m), we continue to undertake research and will resume product development work once we have secured the next round of funds”
“Looking ahead, once we have secured the next round of funding, we plan to bring the product up to industry standard and manufacture on a larger scale, potentially in Singapore.”
“The goal is to obtain the relevant regulatory approvals to enter the US, Asian and African markets. It has CE marking for the UK and European markets and needs to obtain FDA approval for the US and other regulatory approvals for other markets.”

Dr. Shakardokht Jafari, Founder and CTO
TRUEinvivo
Close